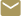Implantable and wearable medical devices such as pacemakers, defibrillators, glucose monitors, nerve stimulators, and drug-infusion pumps are increasingly being used to improve human health. The Georgia Tech Research Institute’s (GTRI) Medical Device Test Center helps protect these types of devices from electromagnetic interference generated by such equipment as inventory control systems, security and logistical systems, RFID systems, metal detectors – and even other medical devices.

Established in the 1960s to help manufacturers protect early heart pacemakers against interference from radar equipment and microwave ovens, the Center today has tested thousands of devices for more than 75 different companies worldwide. By developing test protocols, evaluating device operation and performance and sharing the resulting data with makers of the medical devices and emitting equipment, GTRI researchers help prevent unexpected interactions that could potentially harm patients.
“We work with companies ranging from startups to the largest medical device companies in the world,” said Ralph Herkert, principal research engineer and the Center’s director. “We help manufacturers gain knowledge about how their devices function in the electromagnetic environments where the devices are used by patients. We’re an independent third party that works with not only the medical device manufacturers, but also with the companies that make the emitters.”
Research Grew from Expertise in Electromagnetic Environmental Effects
The Center’s work grew out of GTRI’s long-standing research in electromagnetic environmental effects created by radars, sensors, communication equipment and other radio frequency systems. As those technologies came to be used more widely in public environments, GTRI tapped its expertise to help protect medical devices from the newly created electromagnetic fields. For the early pacemakers, the fields included emissions from commercial microwave ovens, which were designed without knowledge of how the two types of equipment might interact.

Based on test results, manufacturers of pacemakers, which are designed to help ensure consistent heartbeat, put sensitive electronics into metal enclosures and filtered out the unwanted signals that could arise in the devices’ leads (which consist of wires and electrodes) going to the heart. Oven makers installed latches and seals to prevent microwave energy leakage. Largely as a result of this work, microwave ovens today no longer must carry signs warning pacemaker users.
Watch a video showing testing work in the Center
Electronic article surveillance (EAS) systems rely on electromagnetic fields to detect when unpurchased items are being taken from a retail store. GTRI researchers helped manufacturers of the equipment make them more compatible to protect patients using implanted medical devices as they walk past the EAS pillars and gate-like structures at store exits. The Center has similarly evaluated the potential effects of metal detectors used at airports and in public buildings, and warehouse systems that use RFID tags.

“Developers of these devices may not know about some of the concerns from the external environment,” Herkert said. “Since we have relationships with manufacturers of all kinds of different devices, we can help developers look at those concerns and test devices in situations similar to the ways they would be used to see if there might be any concerns.”
Multiple Medical Devices Can Raise Concerns
The expansion of implantable and wearable medical devices has made the situation more complex, creating concerns about interference between two or more devices that a single patient may be using.

“We are now asking whether two or more devices can coexist, doing the functions they were designed to do independently but not interfering with each other to create some new problem or preventing one or both of the devices from being able to do what its developers intended,” Herkert said. “There are a lot of new devices coming onto the market, and all of them must be tested for potential electromagnetic effects from their operation.”
Testing Simulates Conditions in the Human Body
To study the potential interactions between devices, Herkert’s team uses tanks of saline solution to simulate conditions in the human body – usually the torso – in which the prototype devices may be operating. If the concern is implantable devices moving near an EAS system, for example, the devices are submerged in the saline as they operate and moved on a cart past the surveillance system. Center researchers can then study whether output from the medical device changes due to the proximity.

To study interactions between two devices that may be implanted in the body, Herkert places them near each other in a container also filled with saline, which has much the same impedance properties as fluids in the human body. Potential interactions can then be studied and reported to the manufacturers.
“Interactions can range from non-threatening to the patient to something that could be clinically significant,” Herkert said. “We usually don’t make judgements on that, but we tell the manufacturers what the devices are doing and they can determine what actions may need to be taken.” Test results are considered proprietary and shared only with the manufacturers to guide improvements.
Manufacturers may respond by reducing the sensitivity of their devices, adding electronic filters to exclude interfering signals, enclosing the devices in metal shielding, reducing power levels, and taking other steps to improve compatibility with the electromagnetic environment. Demonstrating resistance to interference is part of the approval process devices must undergo before they can be used with patients.
Adapting to the Changing Electromagnetic World
As technology advances for both medical devices and RF-emitting systems, the center’s testing must follow the new trends. For example, advanced leadless pacemakers can be smaller and lighter, and employ either direct connection and/or wireless technologies rather than traditional leads to monitor and assist the heart. These types of advances could create new and potentially challenging interactions, so they must be evaluated.
“There are always going to be new things for us to look at,” Herkert said. “It’s not just the medical devices themselves, but it’s the environments in which they have to operate because they change, too. For example, twenty years ago, we didn’t have to deal with expansion and advances in cellular phones, which will continue to add new capabilities that quickly become ubiquitous.”
As improvements are made in existing devices, they must be tested to make sure the new technology isn’t creating new issues. Modern digital phones can create interactions with hearing aids, for instance, which have to be designed to handle the signals, Herkert said. Powerful magnets that certain manufacturers use to align phones with wireless charging stations can create potential interactions with medical devices. These issues are now recognized and better understood.
New standards are constantly evolving to ensure compatibility between medical devices and emitting equipment, and other laboratories and test centers are taking up some of the responsibilities for testing. Still, Herkert expects GTRI will continue to play a vital role in ensuring that patients can rely on their medical devices.
“We’ll always have a role in doing what we can do to help both the manufacturers of medical devices and the makers of other emitting systems whose technologies are also advancing,” he said. “We need to keep ahead of the standards and new and emerging equipment that medical devices can interact with. The challenges will continue to grow and become more complicated as time goes on.”
Writer: John Toon (john.toon@gtri.gatech.edu)
GTRI Communications
Georgia Tech Research Institute
Atlanta, Georgia USA
The Georgia Tech Research Institute (GTRI) is the nonprofit, applied research division of the Georgia Institute of Technology (Georgia Tech). Founded in 1934 as the Engineering Experiment Station, GTRI has grown to more than 2,900 employees, supporting eight laboratories in over 20 locations around the country and performing more than $940 million of problem-solving research annually for government and industry. GTRI's renowned researchers combine science, engineering, economics, policy, and technical expertise to solve complex problems for the U.S. federal government, state, and industry.